Unveiling Therapeutic Prospects: Evaluating the Anti-Arthritic Properties of Thymosin Alpha 1
Abstract
Background: Thymosin alpha 1 is a peptide naturally occurring in the thymus that has long been recognized for modifying, enhancing, and restoring immune function.
Purpose: The present study focuses on the effects of Thymosin alpha 1 on Body weight, Paw weight, paw volume and arthritic score of Collagen induced arthritis (CIA) rats.
Methods: The wistar rats were randomly divided into 5 groups: nor-mal control, arthritic control, CIA+Ta-1 0.25mg/kg, CIA+Ta-1 0.5mg/kg and CIA+Ta-1 mg/kg. The collagen and lipopolysaccharide (LPS) were administered via the intraplantar route to the experimental rat subjects. Prior to the administration of collagen and LPS, the initial paw volume was measured, and the body weight was duly document-ed. The administration of the treatment occurred on the first, third, and fifth days. On the completion of the dosing period, specifically on the fifteenth day, the final body weight was duly recorded. The measurements of paw volume, paw weight, Body weight and arthritic score were duly recorded.
Results: Following with the administration of Thymosin alpha-1 the arthritic rats shown significantly reduce the severity of arthritis by decreasing the symptoms of arthritis.
Conclusions: Thymosin alpha-1 has shown a promising effect of the reduction in the pathophysiology of rheumatoid arthritis and hence it can be a better therapeutic candidate of future for the management of RA. The more detailed analysis of the mechanistic study may provide a better understanding
-
Page Number : 13-18
-
Published Date : 2023-10-13
-
Keywords
Rheumatoid arthritis, Thymosin alpha-1, CIA rats, Paw weight, Arthritic score -
DOI Number
10.15415/jmrh.2023.101002 -
Authors
Indu Bala and Pranav Kumar Prabhakar
References
- Bax, M., van Heemst, J., Huizinga, T. W., & Toes, R. E. (2011). Genetics of rheumatoid arthritis: what have we learned? Immunogenetics, 63, 459-466.
- Brand DD, Latham KA, Rosloniec EF. (2007). Collagen induced arthritis. Nature Protocol 2:1269–75.
- Conteas, C. N., Mutchnick, M. G., Palmer, K. C., Weller, F. E., Luk, G. D., Naylor, P. H., ... & Horecker, B. L. (1990). Cellular levels of thymosin immunoreactive peptides are linked to proliferative events: evidence for a nuclear site of action. Proceedings of the National Academy of Sciences, 87(9), 3269-3273.
- Dhawan, S. S., & Quyyumi, A. A. (2008). Rheumatoid arthritis and cardiovascular disease. Current atherosclerosis reports, 10(2), 128–133. https://doi.org/10.1007/s11883-008-0019-x
- Egan CG, Lockhart JC, Ferrell WR. (2004). Pathophysiology of vascular dysfunction in a rat model of chronic joint inflammation. J Physiol 557:635–45.
- Fiorentino PM, Talents RH, Miller JN, et al. (2008). Spinal interleukin-1b in a mouse model of arthritis and joint pain. Arthritis Rheum 58:3100–9.
- Gomez-Marquez, J., Segade, F., Dosil, M., Pichel, J. G., Bustelo, X. R., & Freire, M. (1989). The expression of prothymosin α gene in T lymphocytes and leukemic lymphoid cells is tied to lymphocyte proliferation. Journal of Biological Chemistry, 264(15), 8451-8454.
- Granado M, Preigo T, Lopez- Calderon A, et al. (2005). Anti-inflammatory effect of the ghrelin agonist growth hormone-releasingpeptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab288:486–92.
- Gupta, A., & Singh, S. (2014). Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharmaceutical biology, 52(3), 308-320.
- Jevremovic, M., Kartaljevic, G., Jelusic, V., Vodnik, T., Pesic, M., & Filipovic, S. (1997). Determination of thymosin alpha1 with enzyme-immunoassay in colorectal cancer patients. Archive of Oncology, 5(4), 193-194.
- Kremer JM. (2001). Rational use of new and existing disease modifying agents in rheumatoid arthritis. Ann Intern Med 134:695–706.
- Kuijper, T. M., Lamers-Karnebeek, F. B., Jacobs, J. W., Hazes, J. M., &Luime, J. J. (2015). Flare rate in patients with rheumatoid arthritis in low disease activity or remission when tapering or stopping synthetic or biologic DMARD: a systematic review. The Journal of rheumatology, 42(11), 2012-2022.
- Kumar N, Singh S, Patro N, Patro I. (2009). Evaluation of protective efficacy of Spirulina platensis against collagen induced arthritis in rats. Inflammo pharmacology 17:181–90.
- Moreland LW, Russel AS, Paulus HE. (2001). Management of rheumatoid arthritis: The historical context. J Rheumatol 28:1431–52.
- Pica, F., Chimenti, M. S., Gaziano, R., Buè, C., Casalinuovo, I. A., Triggianese, P., ... & Garaci, E. (2016). Serum thymosin α 1 levels in patients with chronic inflammatory autoimmune diseases. Clinical & Experimental Immunology, 186(1), 39-45.
- Rasool M, Sabina EP, Lavanya B. (2006). Anti-inflammatory effect of Spirulina fusiformis on adjuvant-induced arthritis in mice. Biol Pharm Bull 29:2483–7.
- Sewerin, P., Vordenbaeumen, S., Hoyer, A., Brinks, R., Buchbender, C., Miese, F., ... & Ostendorf, B. (2017). Silent progression in patients with rheumatoid arthritis: is DAS28 remission an insufficient goal in RA? Results from the German Remission-plus cohort. BMC musculoskeletal disorders, 18, 1-9.
- Shejawal, N., Menon, S., & Shailajan, S. (2014). A simple, sensitive and accurate method for rat paw volume meas urement and its expediency in preclinical animal studies. Human & experimental toxicology, 33(2), 123-129.
- Smolen, J. S., Landewé, R. B., Bijlsma, J. W., Burmester, G. R., Dougados, M., Kerschbaumer, A., ... & Van Der Heijde, D. (2020). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Annals of the rheumatic diseases, 79(6), 685-699.
- Toh, M. L., &Miossec, P. (2007). The role of T cells in rheumatoid arthritis: new subsets and new targets. Current opinion in rheumatology, 19(3), 284-288.
- Weyand, C. M., & Goronzy, J. J. (1997). Pathogenesis of rheumatoid arthritis. Medical Clinics, 81(1), 29-55.
- Zhang, Q., Tang, D., & Zhao, H. (2010). Immunological therapies can relieve aromatase inhibitor-related joint symptoms in breast cancer survivors. American journal of clinical oncology, 33(6), 557-560.
Introduction
Rheumatoid arthritis, often known as RA, is a chronic autoimmune illness that affects multiple body systems. Rheumatoid arthritis is characterized by the development of persistent inflammatory synovitis, which typically affects peripheral joints in a symmetrical manner (Fiorentino et al., 2008). In certain circumstances, patients also experience extra-articular and systemic signs. Rheumatoid arthritis affects between approximately 0.5 and 1% of the general population in developed nations (Bax et al., 2011). Although disease can strike people of any age, women are nearly three times more likely to be affected than males, and the onset of symptoms typically occurs when a person is in their 40s or 50s, even though disease can strike people of any age. In patients with RA, cardiovascular disease (also known as CVD), which develops as a result of chronic inflammation, is regarded as one of the primary reasons for patient mortality (Dhawan et al., 2008). In RA, Synovial inflammation persists over time, leading to the wear and tear of joints and cartilage. There is mounting evidence that immunological dysfunctions linked to a preponderance of pro-inflammatory responses underlie the chronicity of arthritis. It appears that both regional and systemic abnormalities in the natural defences of our body may have a function in the onset and progression of the disease. Initiation and maintenance of the chronic inflammation in RA are mostly dependent on helper T cells (Th cells), according to a recent study (Weyand et.al., 1997). T cells equipped with CD4 marker on their surface are critical to the pathophysiology of rheumatoid arthritis (Toh et al., 2007), and they are able to differentiate into pro- and anti-inflammatory subpopulations depending on the cytokine microenvironment. Recent advancements in rheumatoid arthritis (RA) pharmaceutical therapy have brought about notable improvements in patient outcomes, promoting remission and enhancing quality of life (Kremer 2001; Moreland et al., 2001). However, the high costs associated with traditional treatments, particularly disease-modifying anti-rheumatic medications (DMARDs), present a significant challenge. The exploration and acceptance of biosimilars, generic substances resembling biological DMARDs, offer a promising alternative to mitigate costs and reduce access disparities. Despite the benefits, concerns persist regarding elevated risks of cardiovascular disease and disease flare-ups (Sewerin et al., 2017; Kuijper et al., 2015). Additionally, therapy failure remains common, emphasizing the need for a deeper understanding of the mechanisms behind treatment toxicity and failure in non-remissive cases. Adverse effects, coupled with the economic barriers, underscore the importance of ongoing research, cost-effective alternatives, and global initiatives to improve patient adherence and overall, RA management (Smolen et al., 2019).
Thymosin alpha 1, initially derived from thymus tissue as a natural substance, is a synthetic peptide consisting of 28 amino acids with an acylated amino-terminal. While early studies utilized a thymic preparation containing approximately 1% thymalfasin, most subsequent research employed synthetically produced Thymosin alpha 1 using solid-phase peptide synthesis. Detectable levels of endogenous thymalfasin in serum range from 0.1 to 1 ng/mL in healthy adults, as measured by immunoassays. Interestingly, diseased individuals tend to exhibit lower circulating concentrations (Jevremovic et al., 1997), while levels are higher during pregnancy. Despite these observations, the source, release mechanisms, and regulation of circulating thymalfasin remain unknown. Thymalfasin is inherent in the sequence of prothymosin, a 126-amino-acid peptide primarily located in the cell nucleus, which has been investigated for its potential impact on cell proliferation (Gomez-Marquez et al., 1990; Conteas et al., 1990). Researchers found that Ta1 might modify immunological activity and had opposing effects on joint complaints in breast cancer survivors (Zhang et al., 2010). Patients with psoriatic arthritis and other chronic inflammatory autoimmune illnesses have been shown to have reduced Ta1 serum levels compared to healthy controls (Pica et al., 2016). The current study was aimed to see the effect of Thymosin alpha-1 on the macroscopic assessment of Collagen induced arthritis.
Methodology
2.1 Experimental model:
The wistar rats, 6-8 weeks of age were purchased from Invivo Biosciences animal house facility, Bengaluru, Karnataka 560091 and were acclimatized in the animal house conditions with a 12:12 h light: dark schedule. Free access to food and water was given ad libitum. Rats were randomly divided into 5 groups: normal control, arthritic control, CIA+ Ta-1 0.25 mg/kg, CIA +Ta-1 0.5 mg/kg and CIA+Ta-1 mg/kg. The collagen and lipopolysaccharide (LPS) were administered via the intraplantar route to the experimental rat subjects. Prior to the administration of collagen and LPS, the initial paw volume was measured, and the body weight was duly documented. The administration of the treatment occurred on the first, third, and fifth days. The brief details about the study given below in Table 1.
Table 1: Brief details about the study
| Species | Rat |
| Strain | Wistar |
| No. of groups | 05 (01 control, 01 disease, 03 for test substance) |
| No. of animals per group | 08 |
| Treatment Age | 6 weeks |
| Identification | By cage card, crystal violet/picric acid body marking |
| Acclimatization | 3 days |
2.2 Dose schedule:
The experimental groups were defined as follows in Table 2:
Table 2: Group Allocation of wistar rats as per the dosage for the anti-arthritic study| Group | Type | Treatment |
| I | Control | Vehicle (I.P.) |
| II | Arthritis group | Type II collagen on day 0 & LPS on day 3 |
| III | Arthritis + Thymosin-α High dose | Type II collagen on day 0 & LPS on day 3 + Thymosin-α of 1 mg/kg (I.P.) on (1st, 3rd & 5th days) |
| IV | Arthritis + Thymosin-α Mid dose | Type II collagen on day 0 & LPS on day 3 + Thymosin-α of 0.5 mg/kg (I.P.) on (1st, 3rd & 5th days) |
| V | Arthritis + Thymosin-α Low dose | Type II collagen on day 0 & LPS on day 3 + Thymosin-α of 0.25 mg/kg (I.P.) on (1st, 3rd & 5th days) |
2.3 Assessment of arthritis and arthritic score:
The evaluation of arthritis and subsequent determination of the arthritic score is a critical undertaking in the realm of medical diagnostics. Arthritis, a condition characterized by inflammation and stiffness of the joints, necessitates a comprehensive assessment to ascertain the severity and impact on the affected individual. The arthritic score was as follows: “Grade 0 = No sign of arthritis”, “Grade 1 = Redness and swelling in paw”, “Grade 2 = Deformity in paw”, “Grade 3 = Ankylosis in paw”, “Grade 4 = Maximal swelling and deformity with ankylosis”. The rats underwent regular screening to observe the onset and progression of arthritis. This screening occurred on a daily basis, commencing on day 0 and continuing until the 14th day. The grading of arthritis severity was determined in accordance with the methodology proposed by Brand et al. (2007). The arthritic score of a rat afflicted with arthritis is determined by calculating the sum of the highest grades of arthritis observed in the affected paws. The data were expressed as the mean ± standard error of the mean (SEM) of eight animals per group.
2.4 Assessment of paw volume:
Prior to the administration of collagen and LPS, the initial paw volume was measured, and the body weight was duly documented. Hind paw edema volume was assessed utilizing a plethysmometer (Insight Ltda. in Ribeirão Preto, Brazil). In brief, this apparatus consists of a compact cylinder containing a buffer, linked to a device designed for quantifying the overall fluid volume. The hind paw of the subject was submerged into the cylinder, and the resultant total volume was measured by calculating the difference between the final and initial volumes, yielding the total paw volume (Shejawal et al., 2014).
2.5 Statistical analysis:Average of all the data was compiled and SEM was calculated. All the parameter of treated groups were compared with negative control group by one-way ANOVA followed by Dunnett’s multiple comparison tests. Values <0.05 were considered as statistically significant.
Results:
The rats, induced with arthritis, underwent a treatment regimen involving the administration of test substances on days 1, 3 and 5, with a consistent timing of test substance application. Throughout the entire study duration, a meticulous daily monitoring process was implemented to assess the overall health of all animals and to identify any discernible clinical changes. Swellings in the hind paw region were consistently observed in both the groups receiving treatment and the induced group. Upon the culmination of the 15-day study period, specifically on the 15th day, a thorough examination was conducted for all animals. This comprehensive assessment encompassed various parameters such as arthritis score, body weight, paw volume and paw weight. This multifaceted approach ensured a comprehensive evaluation of the physiological responses and outcomes associated with the administered treatments and the induced arthritic condition.
3.1 Change in body weight:
As changes in body weight play a significant role in diagnosing rheumatoid arthritis, our study revealed a significant increase in body weight across all treatment groups compared to the disease control. The presented graph illustrates body weight measurements taken on Day-0, signifying the study's onset, and Day-15, marking its conclusion. The data indicates that initially, on day 0, the values of all groups were comparable. However, on the 15th day, a notable decrease in body weight was observed in the arthritic group compared to the control group. Following treatment with thymosin alpha-1, a significant increase in body weight was observed in all treated groups compared to the diseased control group (Figure-1).
The body weight of animals affected by arthritis consistently diminishes throughout the progression of arthritis, as demonstrated by previous studies (Egan et al., 2004; Rasool et al., 2006; Granado et al., 2005). In the current investigation, a substantial decline in body weight was similarly observed in arthritic group compared with control.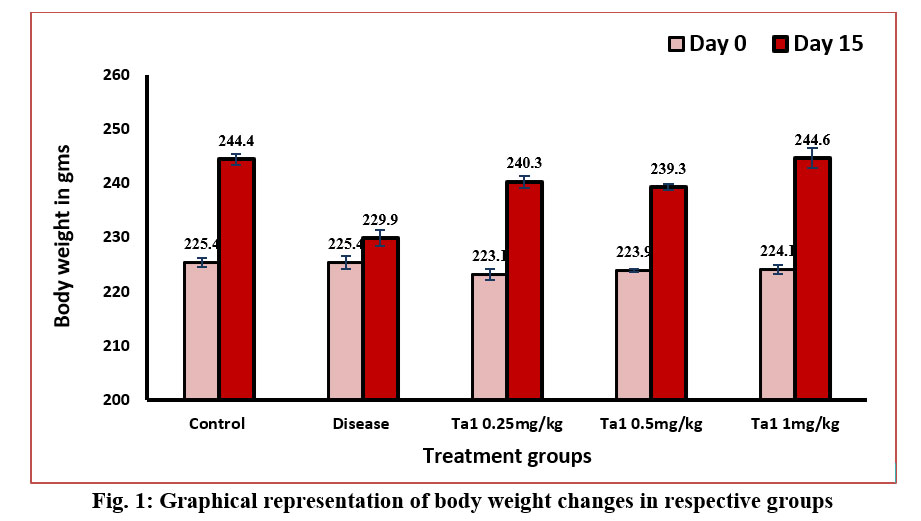
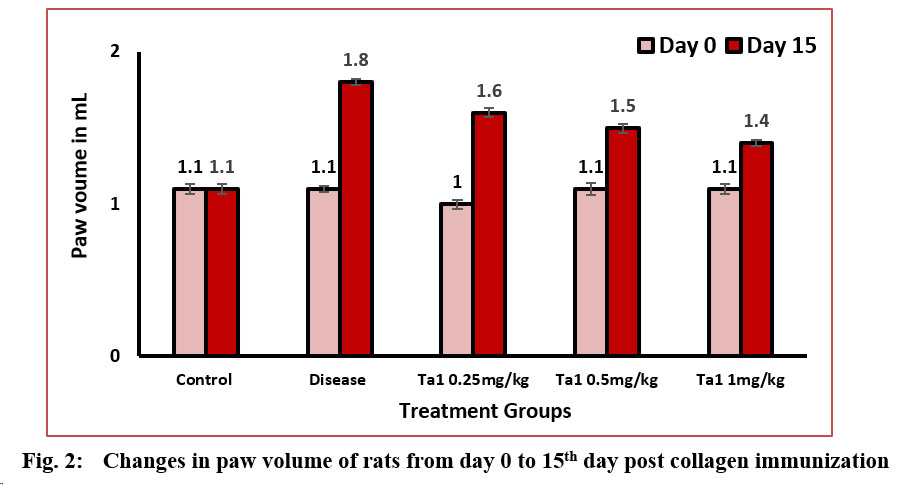
3.2 Change in paw volume:
A conspicuous increase in paw volume was noted in arthritic control rats as arthritis progressed, with measurements on day 0 and the 15th day recorded at 1.1±0.02 and 1.8±0.02 ml, respectively. Arthritic control rats exhibited a significant disparity in paw volume compared to normal control rats, registering values of 1.1±0.03 and 1.1±0.03 ml on day 0 and the 15th day, respectively. Treatment with Thymosin alpha-1 at doses of 0.25 mg/kg, 0.5 mg/kg, and 1 mg/kg resulted in a noteworthy reduction in paw volume in arthritic rats on both day 0 and the 15th day. The values were 1.0±0.03 ml and 1.6±0.03 ml at the dosage of 0.25 mg/kg, 1.1±0.04 ml and 1.5±0.03 ml at the dosage of 0.5 mg/kg, and 1.1±0.03 ml and 1.4±0.02 ml at the dosage of 1 mg/kg. A substantial decrease in paw volume was evident in all treatment groups compared to the disease control group (Figure-2).
3.3 Change in paw weight:
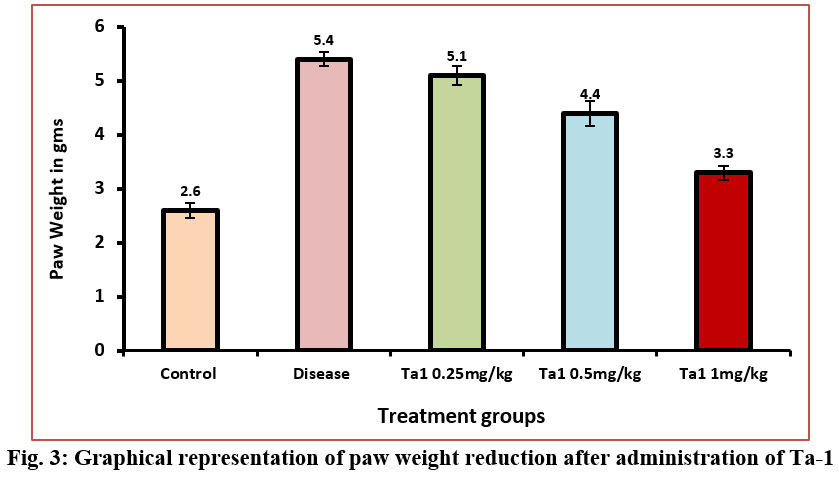
A marked increase in paw weight was observed in arthritic control rats as arthritis advanced, with measurements on the 15th day registering at 5.4±0.13g. Arthritic control rats displayed a significant discrepancy in paw weight compared to normal control rats, with values recorded at 2.6±0.14g on the 15th day. Upon treatment with Thymosin alpha-1 at varying doses (0.25 mg/kg, 0.5 mg/kg, and 1 mg/kg), a notable reduction in paw volume in arthritic rats was evident on day 15th. Specifically, the paw weights were 5.1±0.17g at the dosage of 0.25 mg/kg, 4.4±0.23g at the dosage of 0.5 mg/kg, and 3.3±0.13g at the dosage of 1 mg/kg. A notable reduction in paw weight was observed in the mid and high dosage groups when compared to the arthritis control group (Figure-3). The significant alterations in paw weight underscore the efficacy of Thymosin alpha-1 in mitigating the progression of arthritis in this experimental context.
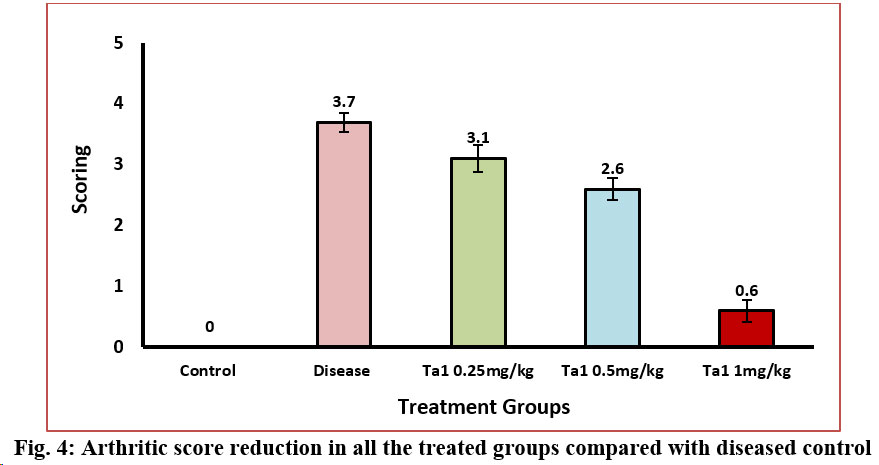
3.4 Arthritic Score:
In arthritic control rats, on day 15th (3.75±0.16) after the arthritis development, a notable arthritic score was observed. However, the treatment of arthritic rats with thymosin alpha-1 (0.25 mg/kg, 0.5 mg/kg and 1 mg/kg) in all three dosage groups led to a significant decrease in their arthritic score on the 15th day (3.12±0.22, 2.62±0.18 and 0.62±0.18) after arthritis development compared to the diseased control group. There was significant decrease in arthritis score of all the treatment groups when compared against disease control (Figure-4). The arthritic score of CIA rats were decreased significantly when treated with W. somnifera (800 mg/kg) and methotrexate (0.3 mg/kg) comparison to their counterparts (Gupta et al., 2014).
Discussion:
RA, a prominent systemic autoimmune disorder, is characterized by persistent synovial inflammation, resulting in joint and cartilage deterioration. Recent advancements in RA pharmaceutical therapy have enabled many patients to achieve remission, enhancing their quality of life and minimizing long-term consequences. Early intervention and continuous care play pivotal roles in reducing damage. However, a notable challenge is the high cost associated with traditional RA treatments, primarily stemming from disease-modifying anti-rheumatic medications (DMARDs) and selective synthetic DMARDs. Despite the benefits, concerns arise, including an elevated risk of cardiovascular disease and disease flare-ups. A recent focus has been on the exploration and acceptance of biosimilars, generic substances resembling biological DMARDs. Some studies suggest their effectiveness, providing a significant alternative to cut costs, broaden treatment options, and reduce access disparities between affluent and developing nations. Even after exhausting therapeutic options, the common occurrence of therapy failure in RA patients emphasizes the need for novel treatments and understanding the mechanisms behind therapy toxicity and failure in non-remissive cases. Adverse effects, coupled with the high cost, pose significant barriers to patient adherence to medications. In rheumatoid arthritis (RA), individuals commonly experience systemic symptoms such as fatigue, fever, and weight loss. Throughout the progression of arthritis in animals, there is a notable and consistent decline in body weight, as indicated by studies conducted by (Egan et al., 2004; Rasool et al., 2006; Gupta et al., 2014). The current study also observed a significant reduction in body weight in arthritic rats when compared to their normal control counterparts. However, arthritic rats treated with Thymosin alpha-1 at all the three dose groups of 0.25 mg/kg, 0.5 mg/kg and 1 mg/kg exhibited a considerable increase in body weight compared to their arthritic control counterparts. This parallels findings in collagen-induced arthritis (CIA) rats, where the administration of Spirulina platensis and Spirulina fusiformis resulted in a similar augmentation of body weight, as reported by (Kumar et al., 2009; Rasool et al., 2006).
Conclusion:
Arthritic rats treated with Thymosin alpha-1 exhibited a notable decrease in their arthritic score, indicating its efficacy in managing inflammation and preventing bone erosion. Overall, the data showed that after administering Thymosin alpha-1 treatment, noticeable and significant changes were observed in various parameters, including body weight, paw volume, paw weight and arthritic score in all the treated groups. Thus, it can be concluded that Thymosin alpha-1 can be used in the treatment of RA and other bone related disorders.
Competing interests:
None declared
Ethics declaration:
The ethical clearance for performing the study was obtained from the university via approval number: Invivo/006/2022.